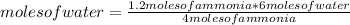Chemistry, 18.04.2021 09:10 samiiegarciia
Given the reaction: 4 NH3(g) + 5 O2(g) → 4 NO (g) + 6 H2O When 1.20 mole of ammonia reacts, how many moles of water are produced?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 05:30
For the reaction i2(g)+br2(g)←−→2ibr(g), kc=280 at 150 ∘c. suppose that 0.450 mol ibr in a 2.00-l flask is allowed to reach equilibrium at 150 ∘c. what is the equilibrium concentration of 2ibr, i2, br2
Answers: 1

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 1

Chemistry, 23.06.2019 13:40
Which of the following volumes is the smallest? a) one microliter b)one deciliter d)one liter c)one milliliter
Answers: 2
You know the right answer?
Given the reaction: 4 NH3(g) + 5 O2(g) → 4 NO (g) + 6 H2O When 1.20 mole of ammonia reacts, how many...
Questions

Mathematics, 06.05.2021 15:50



Computers and Technology, 06.05.2021 15:50





Mathematics, 06.05.2021 15:50

Mathematics, 06.05.2021 15:50

Social Studies, 06.05.2021 15:50

Mathematics, 06.05.2021 15:50


Mathematics, 06.05.2021 15:50



Mathematics, 06.05.2021 15:50

Mathematics, 06.05.2021 15:50

Mathematics, 06.05.2021 15:50

English, 06.05.2021 15:50