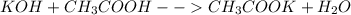Chemistry, 18.04.2021 02:10 firenation18
A 15.5 mL sample of 0.215 M KOH solution required 21.2 mL of aqueous acetic acid solution in a titration experiment. Calculate the molarity of the acetic acid solution.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Write the complete balanced equation for the reaction between lead (iv) oxide (pbo2) and water (h2o).
Answers: 1


Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 23.06.2019 00:30
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2
You know the right answer?
A 15.5 mL sample of 0.215 M KOH solution required 21.2 mL of aqueous acetic acid solution in a titra...
Questions


SAT, 04.11.2020 21:00

Mathematics, 04.11.2020 21:00

Mathematics, 04.11.2020 21:00


Mathematics, 04.11.2020 21:00


History, 04.11.2020 21:00

Mathematics, 04.11.2020 21:00


English, 04.11.2020 21:00



Mathematics, 04.11.2020 21:00

Mathematics, 04.11.2020 21:00



Mathematics, 04.11.2020 21:00

Mathematics, 04.11.2020 21:00