Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3


Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
You know the right answer?
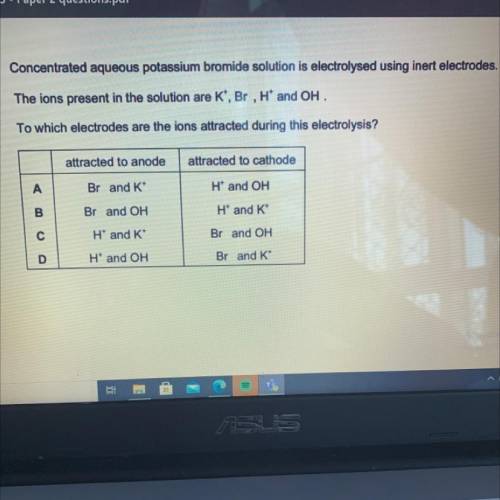
Concentrated aqueous potassium bromide solution is electrolysed using inert electrodes.
The ions pr...
Questions


History, 14.02.2020 07:04

History, 14.02.2020 07:04




Mathematics, 14.02.2020 07:05

Biology, 14.02.2020 07:06

Mathematics, 14.02.2020 07:06

Biology, 14.02.2020 07:06

Mathematics, 14.02.2020 07:06




Mathematics, 14.02.2020 07:17

Physics, 14.02.2020 07:19

Mathematics, 14.02.2020 07:19


Mathematics, 14.02.2020 07:19

English, 14.02.2020 07:20