
Chemistry, 17.04.2021 02:50 serenitynycole
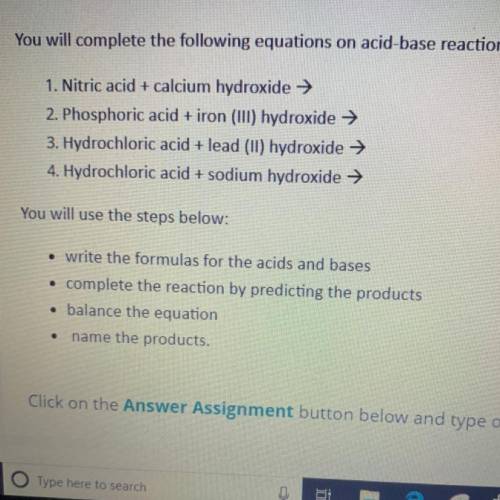
You will complete the following equations on acid-base reactions.
1. Nitric acid + calcium hydroxide →
2. Phosphoric acid + iron (III) hydroxide →
3. Hydrochloric acid + lead (11) hydroxide →
4. Hydrochloric acid + sodium hydroxide →
You will use the steps below:
• write the formulas for the acids and bases
• complete the reaction by predicting the products
• balance the equation
name the products.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
You will complete the following equations on acid-base reactions.
1. Nitric acid + calcium hydroxid...
Questions

Social Studies, 02.03.2021 21:40

Computers and Technology, 02.03.2021 21:40




Mathematics, 02.03.2021 21:40

Mathematics, 02.03.2021 21:40

English, 02.03.2021 21:40


Mathematics, 02.03.2021 21:40


English, 02.03.2021 21:40


English, 02.03.2021 21:40

Social Studies, 02.03.2021 21:40

English, 02.03.2021 21:40

Biology, 02.03.2021 21:40

Mathematics, 02.03.2021 21:40

Mathematics, 02.03.2021 21:40



