
Chemistry, 17.04.2021 01:00 Albraaalouda
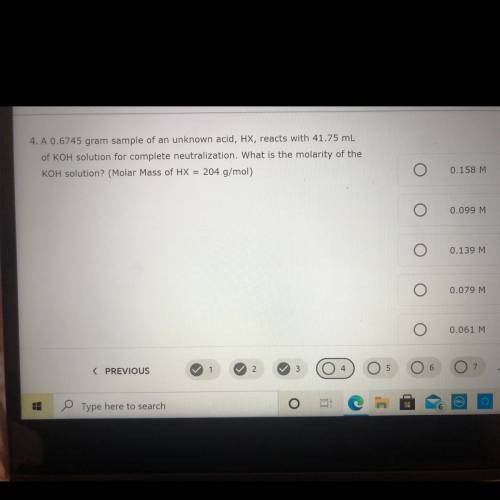
is 0.6745 g sample of an unknown acid HX reacts with 41.75 mL of KOH solution for complete neutralization. What is the molarity of the KOH solution

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
You know the right answer?
is 0.6745 g sample of an unknown acid HX reacts with 41.75 mL of KOH solution for complete neutraliz...
Questions

Mathematics, 28.02.2021 01:00



Biology, 28.02.2021 01:00

Mathematics, 28.02.2021 01:00








Biology, 28.02.2021 01:00

Mathematics, 28.02.2021 01:00


Mathematics, 28.02.2021 01:00

Mathematics, 28.02.2021 01:00



History, 28.02.2021 01:00



