
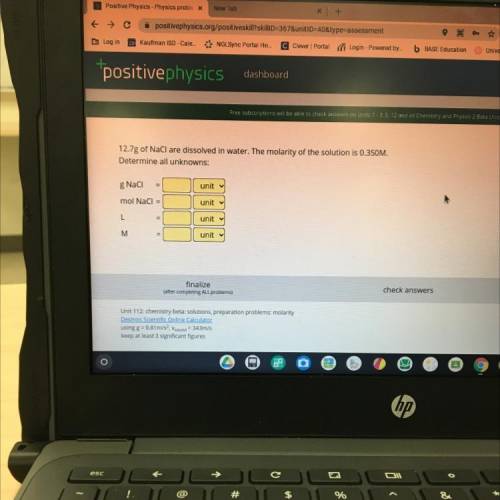
12.7g of NaCl are dissolved in water. The molarity of the solution is 0.350M.
Determine all unknowns:
g Naci
mol NaCl =
unity
L
unit
M
unity
finalize
(after completing ALL problems)
check answers
Unit 112: chemistry beta: solutions, preparation problems: molarity
Desmos Scientific Online Calculator

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1



Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
12.7g of NaCl are dissolved in water. The molarity of the solution is 0.350M.
Determine all unknown...
Questions

Mathematics, 04.08.2021 19:20



Mathematics, 04.08.2021 19:20

Mathematics, 04.08.2021 19:20



Social Studies, 04.08.2021 19:20


Business, 04.08.2021 19:20

English, 04.08.2021 19:20



Chemistry, 04.08.2021 19:20




Chemistry, 04.08.2021 19:30

Computers and Technology, 04.08.2021 19:30

Mathematics, 04.08.2021 19:30



