
Chemistry, 16.04.2021 20:40 homeschool0123
An analytical chemist is titrating 111.0 mL of a 0.3700 M solution of aniline (C6H5NH2) with a 0.3500 M solution of HNO3. The pK_b of aniline is 9.37. Calculate the pH of the base solution after the chemist has added 79.1 mL of the HNO_3 solution to it.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3
You know the right answer?
An analytical chemist is titrating 111.0 mL of a 0.3700 M solution of aniline (C6H5NH2) with a 0.350...
Questions

Mathematics, 16.04.2021 14:00

History, 16.04.2021 14:00



Mathematics, 16.04.2021 14:00



Mathematics, 16.04.2021 14:00



Mathematics, 16.04.2021 14:00

Spanish, 16.04.2021 14:00

English, 16.04.2021 14:00


Biology, 16.04.2021 14:00

World Languages, 16.04.2021 14:00

History, 16.04.2021 14:00



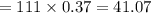
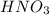 millimoles
millimoles 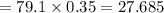
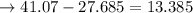 millimoles aniline left
millimoles aniline left
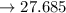 millimoles salt formed
millimoles salt formed
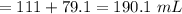
![\to [aniline] = \frac{13.385}{190.1} = 0.07 \ M\\\\\to [salt] =\frac{ 27.685}{ 190.1} = 0.146\ M\\\\\to pOH = pKb + \frac{\log [salt]}{ [base]}\\\\\to pOH = 9.37 + \frac{\log [0.146]}{[0.07]}\\\\\to pOH = 9.69\\\\\to pH = 14 - 9.69\\\\\to pH = 4.31\\](/tpl/images/1264/8201/7a097.png)


