
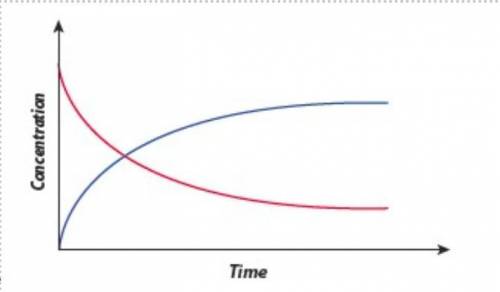
This graph shows changes in concentration during a chemical reaction. Use the definition of reaction rate to explain which curve represents the reactants and which curve represents the products. Explain the shape of each line. What is happening to the reaction rate as the reactant concentration is changed? What is happening where the two lines cross?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2

Chemistry, 23.06.2019 07:00
Under what conditions will a gas be most likely to exhibit the ideal gas properties predicted by the ideal gas law? 1)high pressures and high temperature, because particles are forced closer together with higher kinetic energy, so intermolecular forces of attraction are weaker 2)high pressure and low temperature, because particles are forced closer together and moving slower, so the volume of the particles is less significant 3) low pressure and high temperature, because particles are spread farther apart and moving faster, so the intermolecular forces of attraction are weaker 4)low pressure and low temperature, because particles are spread farther apart with lower kinetic energy, so the volume of the particles is less significant
Answers: 2
You know the right answer?
This graph shows changes in concentration during a chemical reaction. Use the definition of reaction...
Questions

Mathematics, 18.05.2021 01:30

Mathematics, 18.05.2021 01:30

Mathematics, 18.05.2021 01:30

Computers and Technology, 18.05.2021 01:30


History, 18.05.2021 01:30




Mathematics, 18.05.2021 01:30



History, 18.05.2021 01:30

Health, 18.05.2021 01:30

History, 18.05.2021 01:30


Mathematics, 18.05.2021 01:30

English, 18.05.2021 01:30


Mathematics, 18.05.2021 01:30



