
Chemistry, 16.04.2021 17:30 SiegeHatake4534
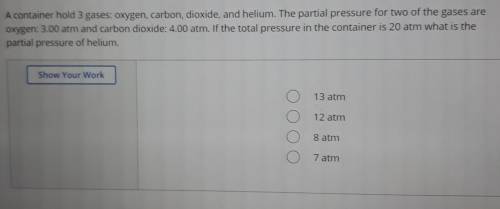
A container hold 3 gases: oxygen, carbon, dioxide, and helium. The partial pressure for two of the gases are oxygen: 3.00 atm and carbon dioxide: 4.00 atm. If the total pressure in the container is 20 atm what is the partial pressure of helium.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
You know the right answer?
A container hold 3 gases: oxygen, carbon, dioxide, and helium. The partial pressure for two of the g...
Questions


Mathematics, 12.08.2020 04:01

Biology, 12.08.2020 04:01







Mathematics, 12.08.2020 04:01

Biology, 12.08.2020 04:01



Mathematics, 12.08.2020 04:01

Mathematics, 12.08.2020 04:01



Business, 12.08.2020 04:01

English, 12.08.2020 04:01



