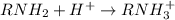Chemistry, 16.04.2021 03:20 makaylahunt
According to the Lewis model of acids and bases, amines behave as bases in water because . View Available Hint(s) According to the Lewis model of acids and bases, amines behave as bases in water because . the hydrogens will bond with hydroxide ions to make water they produce hydroxide ions they donate the hydrogens they have as hydrogen ions the unshared pair of electrons on the nitrogen can accept a proton

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
There is an area in idaho named craters of the moon where most of the ground is covered with basalt, adark gray, igneous rock with no visibl crystals. what can you infer about the geographical history of the area?
Answers: 1

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
According to the Lewis model of acids and bases, amines behave as bases in water because . View Avai...
Questions

Geography, 17.05.2021 17:10


Computers and Technology, 17.05.2021 17:10

Mathematics, 17.05.2021 17:10

Health, 17.05.2021 17:10


Mathematics, 17.05.2021 17:10

Computers and Technology, 17.05.2021 17:10

English, 17.05.2021 17:10


Mathematics, 17.05.2021 17:10


Mathematics, 17.05.2021 17:10

Mathematics, 17.05.2021 17:10



Mathematics, 17.05.2021 17:10

Mathematics, 17.05.2021 17:10

Mathematics, 17.05.2021 17:10

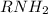 which has lone pair of electrons on nitrogen and thus is able to donate electrons to a lewis acid which is short of electrons. In other words nitrogen can accept a proton.
which has lone pair of electrons on nitrogen and thus is able to donate electrons to a lewis acid which is short of electrons. In other words nitrogen can accept a proton.