
Chemistry, 16.04.2021 01:00 karrathomas
Calcium carbonate decomposes to form calcium oxide and carbon dioxide, like this: (s)(s)(g) At a certain temperature, a chemist finds that a reaction vessel containing a mixture of calcium carbonate, calcium oxide, and carbon dioxide at equilibrium has the following composition: compound amount Calculate the value of the equilibrium constant for this reaction. Round your answer to significant digits.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 22:30
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2
You know the right answer?
Calcium carbonate decomposes to form calcium oxide and carbon dioxide, like this: (s)(s)(g) At a cer...
Questions


Mathematics, 17.01.2021 06:00

Mathematics, 17.01.2021 06:00

English, 17.01.2021 06:00


Mathematics, 17.01.2021 06:00

Mathematics, 17.01.2021 06:00







Mathematics, 17.01.2021 06:00

Mathematics, 17.01.2021 06:00

English, 17.01.2021 06:00

Physics, 17.01.2021 06:00

Mathematics, 17.01.2021 06:00

English, 17.01.2021 06:00

Chemistry, 17.01.2021 06:00

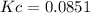
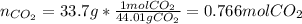
![[CO_2]=\frac{0.766molCO_2}{9.0L}=0.0851M](/tpl/images/1262/6588/3d5ed.png)
![Kc=[CO_2]\\\\Kc=0.0851](/tpl/images/1262/6588/d8c10.png)


