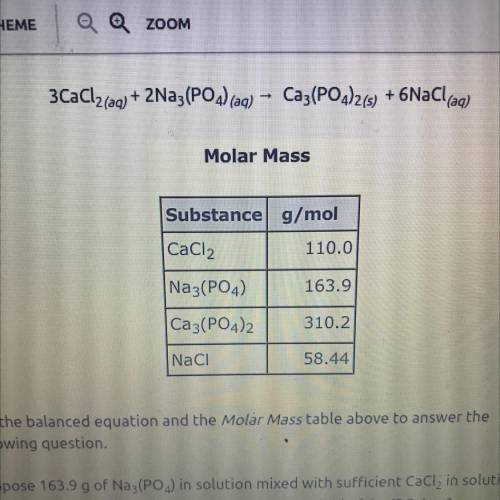
3CaCl2(aq) + 2Na3(PO4) (aq)
Ca3(PO4)2(s) + 6NaCl(aq)
Use the balanced equation and the Molar...

Chemistry, 16.04.2021 01:00 DragonLovely
3CaCl2(aq) + 2Na3(PO4) (aq)
Ca3(PO4)2(s) + 6NaCl(aq)
Use the balanced equation and the Molar Mass table above to answer the
following question.
Suppose 163.9 g of Na3(PO4) in solution mixed with sufficient CaCl, in solution
yields 116 g of Ca3(PO4)2(s). What is the percent yield of Ca3(PO4)2(5)?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1
You know the right answer?
Questions

Mathematics, 17.10.2021 18:30


Biology, 17.10.2021 18:30

Mathematics, 17.10.2021 18:30


Mathematics, 17.10.2021 18:30

Mathematics, 17.10.2021 18:30

Mathematics, 17.10.2021 18:30

Mathematics, 17.10.2021 18:30

English, 17.10.2021 18:30

Mathematics, 17.10.2021 18:30



English, 17.10.2021 18:40


English, 17.10.2021 18:40


Mathematics, 17.10.2021 18:40

Biology, 17.10.2021 18:40

Physics, 17.10.2021 18:40