
Chemistry, 15.04.2021 23:20 taylormjensen
2. Consider the following chemical equation and equilibrium constant at 25oC:
2COF2(g) <--> CO2(g) + CF4(g) K = 2.2 x 10^6
Calculate the equilibrium constant for the following reaction at 25oC:
2CO2(g) + 2CF4(g) <--> 4COF2
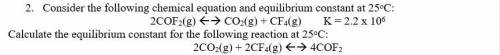

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1


Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3
You know the right answer?
2. Consider the following chemical equation and equilibrium constant at 25oC:
2COF2(g) <--> C...
Questions


Mathematics, 14.02.2020 05:16

Mathematics, 14.02.2020 05:16



Biology, 14.02.2020 05:16



Mathematics, 14.02.2020 05:17


English, 14.02.2020 05:17


Computers and Technology, 14.02.2020 05:17




English, 14.02.2020 05:18



Mathematics, 14.02.2020 05:18



