Why do weak acid-weak base reactions not go to completion?
there are not enough molecul...

Chemistry, 22.11.2019 04:31 jayycruz59
Why do weak acid-weak base reactions not go to completion?
there are not enough molecules in solution to react with each other.
the conjugate products are much weaker than the original acid and base.
neither a weak acid nor a weak base has a strong tendency to transfer h+ ions.
both weak acids and weak bases have a strong tendency to transfer h+ ions, so they cancel each other out.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1


Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
You know the right answer?
Questions



English, 02.12.2020 05:10


History, 02.12.2020 05:10

Mathematics, 02.12.2020 05:10

Social Studies, 02.12.2020 05:10

History, 02.12.2020 05:10



Mathematics, 02.12.2020 05:10


Mathematics, 02.12.2020 05:10



Health, 02.12.2020 05:10

Mathematics, 02.12.2020 05:10


Mathematics, 02.12.2020 05:10

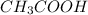 ) is a weak acid and it dissociates poorly in water.
) is a weak acid and it dissociates poorly in water.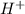 ions.
ions.

