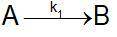Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1

Chemistry, 23.06.2019 16:00
Which part of the mantle is similar to the crust ? (science earth's layers)
Answers: 1
You know the right answer?
Speed constant data against temperature were obtained for the reaction. Calculate the coefficients a...
Questions

Mathematics, 13.01.2021 03:00

Geography, 13.01.2021 03:00

Mathematics, 13.01.2021 03:00




Spanish, 13.01.2021 03:00

English, 13.01.2021 03:00

History, 13.01.2021 03:00


English, 13.01.2021 03:00






Physics, 13.01.2021 03:00



Biology, 13.01.2021 03:00