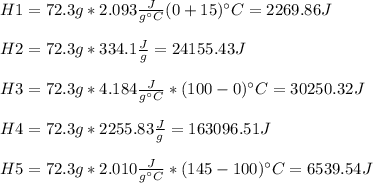Chemistry, 14.04.2021 21:40 deaishaajennings123
72.3 g of ice at -15.0 o C has heat energy added to it until it becomes steam at 145 o C. Calculate the total amount of heat energy needed (in Joules) to accomplish this.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
In which layer of earth do most earthauakes occur a_ inner core b_outer core c_mantle d_crust
Answers: 1

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1
You know the right answer?
72.3 g of ice at -15.0 o C has heat energy added to it until it becomes steam at 145 o C. Calculate...
Questions

Mathematics, 10.10.2019 15:10


Mathematics, 10.10.2019 15:10

History, 10.10.2019 15:10

Mathematics, 10.10.2019 15:10

Mathematics, 10.10.2019 15:10




Mathematics, 10.10.2019 15:10


Health, 10.10.2019 15:10

Biology, 10.10.2019 15:10

English, 10.10.2019 15:10

Mathematics, 10.10.2019 15:10

Physics, 10.10.2019 15:10

History, 10.10.2019 15:10

Mathematics, 10.10.2019 15:10