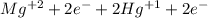Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Which term refers to a property that depends only on the amount of a substance? ©@
Answers: 2

Chemistry, 21.06.2019 19:30
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1

Chemistry, 21.06.2019 20:00
Drag each number to the correct location on the equation. each number can be used more than once, but not all numbers will be used. balance the equation with the coefficients. 2 3 4 5 kclo3 -> kcl + o2
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
You know the right answer?
Balance the following oxidation-reduction reactions, which take place in acidic solution, by using t...
Questions


History, 18.11.2020 22:40


Mathematics, 18.11.2020 22:40

Spanish, 18.11.2020 22:40

Chemistry, 18.11.2020 22:40

Arts, 18.11.2020 22:40

Mathematics, 18.11.2020 22:40


Advanced Placement (AP), 18.11.2020 22:40



Mathematics, 18.11.2020 22:40

Physics, 18.11.2020 22:40

Mathematics, 18.11.2020 22:40


Mathematics, 18.11.2020 22:40

Advanced Placement (AP), 18.11.2020 22:40

Mathematics, 18.11.2020 22:40

Social Studies, 18.11.2020 22:40

 ⇒
⇒ 
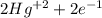 ⇒
⇒ 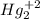
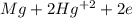 ⇒
⇒