
Chemistry, 28.10.2019 10:31 manywonders
The reaction of chlorine gas with solid phosphorus (p4)produces solid phosphorus pentachloride. when 16.0 g chlorine reacts with 23.0 g p4, which reactant limits the amount of phosphorus pentachloride produced? which reactant is in excess?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3


Chemistry, 23.06.2019 07:30
Assignment directions: pick one of the following chemists and perform a bit of research on him/her. answer the following questions. alice hamilton rosalind franklin marie curie gertrude b. elion ada yonath henry cavendish robert boyle antoine lavoisier mario j. molina svante arrhenius
Answers: 1

Chemistry, 23.06.2019 10:00
State the effect on the concentration of the clo- ion when there is a decrease in the concentration of the oh- ion
Answers: 1
You know the right answer?
The reaction of chlorine gas with solid phosphorus (p4)produces solid phosphorus pentachloride. when...
Questions

History, 08.10.2019 15:30

Social Studies, 08.10.2019 15:30


Social Studies, 08.10.2019 15:30


Mathematics, 08.10.2019 15:30

Mathematics, 08.10.2019 15:30

Chemistry, 08.10.2019 15:30

Biology, 08.10.2019 15:30

Chemistry, 08.10.2019 15:30



Mathematics, 08.10.2019 15:30

Arts, 08.10.2019 15:30



Health, 08.10.2019 15:30

Physics, 08.10.2019 15:30

Chemistry, 08.10.2019 15:30

English, 08.10.2019 15:30

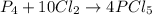
![\frac{23.0 g}{124 g/mol}=0.1854 mol]](/tpl/images/0349/3111/b72f3.png)
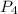 = 4 × 31 g/mol =124 g/mol)
= 4 × 31 g/mol =124 g/mol)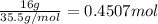
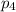 will react with 1.854 mol of chlorine gas.
will react with 1.854 mol of chlorine gas.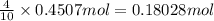 of phosphorus pentachloride.
of phosphorus pentachloride.

