Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1
You know the right answer?
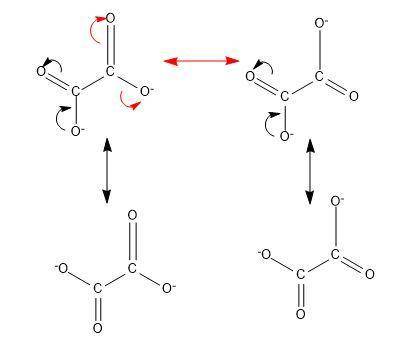
Determine the number of resonance structures for the oxalate ion, c2o42−.
2
...
2
...
Questions

Mathematics, 04.12.2020 18:20


Social Studies, 04.12.2020 18:20

Mathematics, 04.12.2020 18:20

Geography, 04.12.2020 18:20

English, 04.12.2020 18:20


English, 04.12.2020 18:30

Social Studies, 04.12.2020 18:30

Mathematics, 04.12.2020 18:30

Mathematics, 04.12.2020 18:30


History, 04.12.2020 18:30

Mathematics, 04.12.2020 18:30





Mathematics, 04.12.2020 18:30