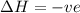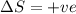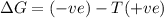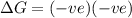Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1


You know the right answer?
What does a process require to be spontaneous at all temperatures? answer a catalyst and lower acti...
Questions


History, 08.10.2021 14:00



Mathematics, 08.10.2021 14:00


Physics, 08.10.2021 14:00

English, 08.10.2021 14:00

Chemistry, 08.10.2021 14:00


History, 08.10.2021 14:00



Mathematics, 08.10.2021 14:00

Mathematics, 08.10.2021 14:00


Computers and Technology, 08.10.2021 14:00

Chemistry, 08.10.2021 14:00

Mathematics, 08.10.2021 14:00

Mathematics, 08.10.2021 14:00

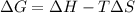
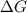 = free energy change
= free energy change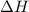 = enthalpy change
= enthalpy change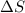 = entropy change
= entropy change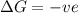 , reaction is spontaneous
, reaction is spontaneous