
Chemistry, 14.04.2021 08:20 zamyapritchard5
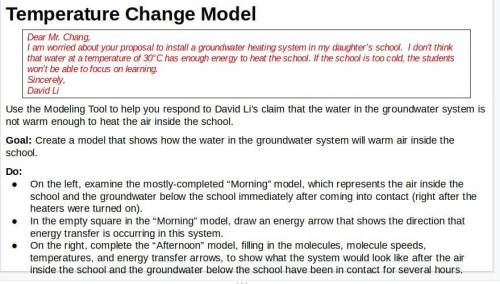
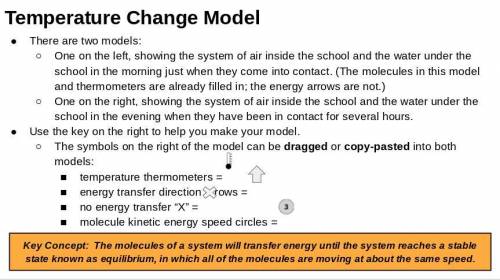
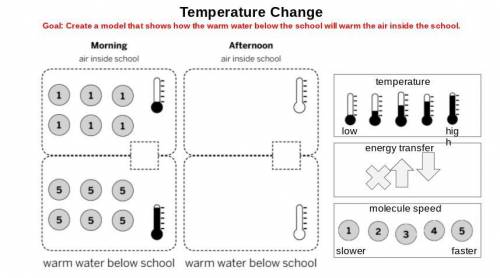
Respond to David Li’s letter. Explain how the groundwater system could heat the air in the school. Explain what would happen to the air temperature at Riverdale School if the groundwater system were used. In addition to the unit vocabulary, be sure to use the terms stability and change in your explanation.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3

Chemistry, 23.06.2019 06:30
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3

Chemistry, 23.06.2019 10:30
What are two radioactive isotopes that are used for dating rocks that are older than 10 million years
Answers: 2
You know the right answer?
Respond to David Li’s letter. Explain how the groundwater system could heat the air in the school....
Questions



English, 05.03.2021 22:40


Chemistry, 05.03.2021 22:40

Social Studies, 05.03.2021 22:40

Mathematics, 05.03.2021 22:40

Chemistry, 05.03.2021 22:40





Mathematics, 05.03.2021 22:40

Mathematics, 05.03.2021 22:40



Computers and Technology, 05.03.2021 22:40



Chemistry, 05.03.2021 22:40



