
Chemistry, 14.04.2021 05:50 carter4026
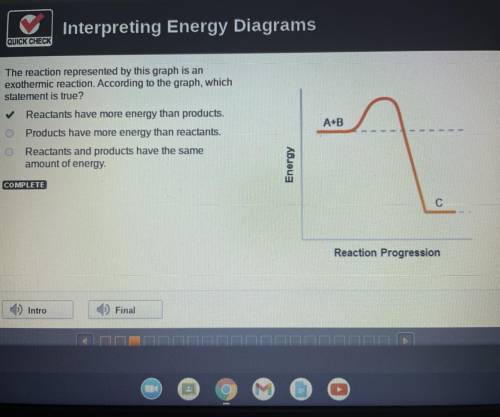
The reaction represented by this graph is an
exothermic reaction. According to the graph, which statement is true?
Reactants have more energy than products.
Products have more energy than reactants.
Reactants and products have the same
amount of energy.
A+B
Energy
COMPLETE
C
Reaction Progression

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
The reaction represented by this graph is an
exothermic reaction. According to the graph, which sta...
Questions

Social Studies, 28.01.2021 21:50


Physics, 28.01.2021 21:50


Mathematics, 28.01.2021 21:50

Computers and Technology, 28.01.2021 21:50




English, 28.01.2021 21:50

Advanced Placement (AP), 28.01.2021 21:50

Mathematics, 28.01.2021 21:50



Mathematics, 28.01.2021 21:50

English, 28.01.2021 21:50

Mathematics, 28.01.2021 21:50

SAT, 28.01.2021 21:50


Mathematics, 28.01.2021 21:50




