Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 23.06.2019 07:00
Why do the strengths of london (dispersion) forces generally increase with increasing molecular size? choose one: a. heavier atoms have stronger attractions for each other than lighter atoms. b. dispersion forces are all equal in magnitude; there is no size dependence. c. dispersion forces arise from the attraction between the nuclei of atoms, and larger molecules have larger nuclei. d. dispersion forces arise from dipoles caused by the electron distribution being distorted. larger molecules have more electrons and, therefore, more distortions and a bigger force. e. dispersion forces depend on distance. larger molecules are farther apart and so the forces are smaller.
Answers: 2
You know the right answer?
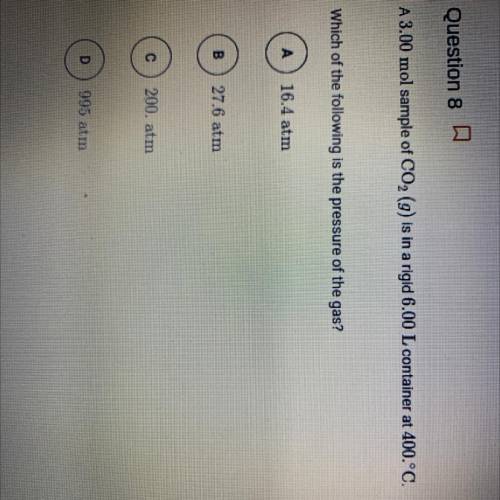
A3.00 mol sample of CO2 (g) is in a rigid 6.00 L container at 400.°C.
Which of the following is the...
Questions



World Languages, 17.04.2021 18:10


Chemistry, 17.04.2021 18:10



Social Studies, 17.04.2021 18:10



Mathematics, 17.04.2021 18:10

Mathematics, 17.04.2021 18:10

Law, 17.04.2021 18:10





Mathematics, 17.04.2021 18:10

Computers and Technology, 17.04.2021 18:10