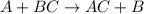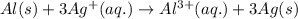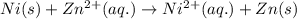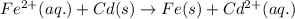Chemistry, 27.09.2019 01:40 ryleerose255
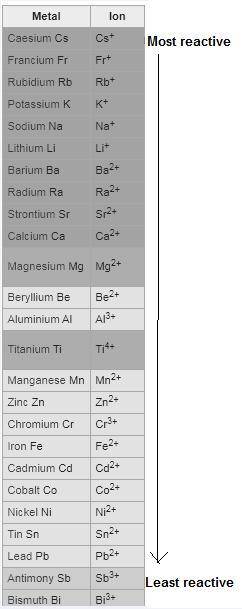
Which of the following redox reactions do you expect to occur spontaneously in the forward direction?
check all that apply.
ni(s)+pb2+(aq)→ni2+(aq)+pb(s)
al(s)+3ag+(aq)→al3+(aq)+3ag(s)
ni(s)+zn2+(aq)→ni2+(aq)+zn(s)
fe2+(aq)+cd(s)→fe(s)+cd2+(aq)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1


Chemistry, 23.06.2019 12:30
If you reacted 450 g of trimethylgallium with 300 g of arsine, what mass of gaas could you make?
Answers: 1
You know the right answer?
Which of the following redox reactions do you expect to occur spontaneously in the forward direction...
Questions



French, 19.07.2019 15:00

Mathematics, 19.07.2019 15:00

Computers and Technology, 19.07.2019 15:00



Mathematics, 19.07.2019 15:00




Chemistry, 19.07.2019 15:00


Mathematics, 19.07.2019 15:00

History, 19.07.2019 15:00

Mathematics, 19.07.2019 15:00