
Chemistry, 13.04.2021 18:20 Trucofer8159
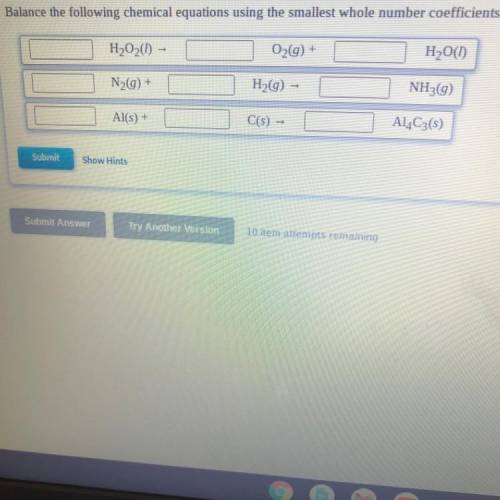
Balance the following chemical equations using the smallest whole number coefficients.
H2O2(I) -
O2(g) +
H2O(1)
N29) +
H2(g) –
NH3(g)
Al(s) +
C(s) -
Al4C3(5)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:20
What is the strongest intermolecular force between an nacl unit and an h2o molecule together in a solution? covalent bonding dipole-dipole force hydrogen bonding ion-dipole force
Answers: 1



Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3
You know the right answer?
Balance the following chemical equations using the smallest whole number coefficients.
H2O2(I) -
Questions



Chemistry, 29.01.2020 07:52

Geography, 29.01.2020 07:52


Mathematics, 29.01.2020 07:52





English, 29.01.2020 07:52

English, 29.01.2020 07:52

Mathematics, 29.01.2020 07:52

English, 29.01.2020 07:52

History, 29.01.2020 07:52

Biology, 29.01.2020 07:52



Mathematics, 29.01.2020 07:52



