
Chemistry, 13.04.2021 08:00 Isaacochoa780
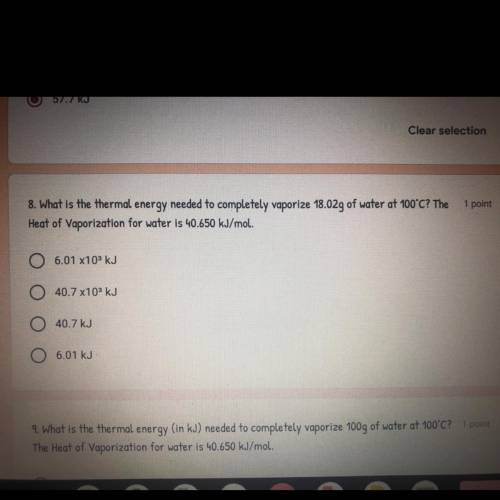
What is the thermal energy needed to vaporize 18.02g of water at 100°C? The Heat of Vaporization for water is 40.650 kJ/mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1


Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2
You know the right answer?
What is the thermal energy needed to vaporize 18.02g of water at 100°C? The Heat of Vaporization for...
Questions



Mathematics, 30.10.2020 03:10



Mathematics, 30.10.2020 03:10

Mathematics, 30.10.2020 03:10

Mathematics, 30.10.2020 03:10




History, 30.10.2020 03:10


History, 30.10.2020 03:10

Advanced Placement (AP), 30.10.2020 03:10

English, 30.10.2020 03:10

Computers and Technology, 30.10.2020 03:10

Mathematics, 30.10.2020 03:10

Biology, 30.10.2020 03:10

Mathematics, 30.10.2020 03:10



