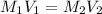Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3


Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1
You know the right answer?
Mr. Whiteman has two stock solutions of sulfuric acid (H2SO4) in the supply closet: 12.1 M and 6.0 M...
Questions


Physics, 08.04.2021 14:00




English, 08.04.2021 14:00

Health, 08.04.2021 14:00

Biology, 08.04.2021 14:00


English, 08.04.2021 14:00


Arts, 08.04.2021 14:00

Business, 08.04.2021 14:00

Biology, 08.04.2021 14:00

World Languages, 08.04.2021 14:00

Mathematics, 08.04.2021 14:00

Engineering, 08.04.2021 14:00

English, 08.04.2021 14:00

Mathematics, 08.04.2021 14:00