
Chemistry, 13.04.2021 02:00 Kkampudiaa
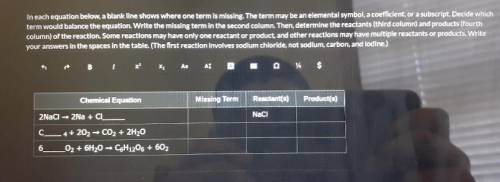
In each term equation below a blank line shows one term missing the terms may be an elemental symbol a coefficient or a subscript decide which term would balance the equation write the missing term in the second column the. Determine the reactants third column and products forth column of the reaction some reactions may have only one reactant or product and other reactions may have only one reactant or product and other reactions may have multiple reactants or products write your answers in the spaces in the table the first reaction involves sodium chloride not sodium carbon and iodine

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:20
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3
You know the right answer?
In each term equation below a blank line shows one term missing the terms may be an elemental symbol...
Questions



Mathematics, 02.03.2021 23:40


Computers and Technology, 02.03.2021 23:40

Mathematics, 02.03.2021 23:40

Mathematics, 02.03.2021 23:40



Mathematics, 02.03.2021 23:40


Mathematics, 02.03.2021 23:40

History, 02.03.2021 23:40

Mathematics, 02.03.2021 23:40

English, 02.03.2021 23:40







