
Chemistry, 13.04.2021 02:10 Misspaige4453
It is time to practice using potential energy diagrams. Respond to the three questions below on energy diagrams and submit to your instructor.
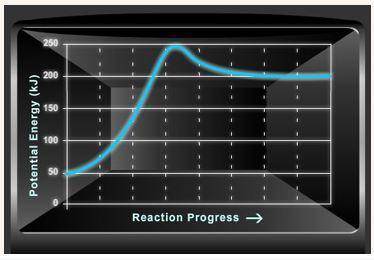
Consider the potential energy diagram shown below. This graph shows the chemical potential energy in a reaction system over time. The y-axis is potential energy in kilojoules. The x-axis is the reaction progress, or time.
Does this graph represent an endothermic or an exothermic reaction? Explain your answer.
What is the enthalpy change, ΔH, for this reaction? Show your work.
What is the activation energy, Ea, for this reaction? Show your work.
In a particular chemical reaction, the energy of the reactants is 30 kJ and the energy of the products is 5 kJ. The maximum energy of the system is 40 kJ.
Sketch a potential energy diagram for this reaction. Make sure to label the energy of the reactants, the energy of the products, the activation energy, and the enthalpy change for the reaction.
What is the activation energy for this reaction?
What is the enthalpy change for this reaction?
Is this reaction endothermic or exothermic? Explain your answer in two ways: first, using the energy values, and second, by referring to the shape of the graph.
The coating on the head of a match is highly flammable. When it burns, it releases a great deal of energy. However, before the match can burn, it must gain a small amount of energy from a spark. That spark is typically produced by striking (rubbing) the match head against a rough surface. Sketch and describe a potential energy diagram that represents the striking and burning of the match. Remember to label the diagram with the energy changes that occur. Your answer must include the potential energy diagram and a written description. (Note: you do not have to use actual energy values.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Actual ingredients of lab (the cookies i am actually making) 1/2 cup sugar 1/2 cup brown sugar 1 1/3 stick margarine 1 egg 1/2 tsp salt 1 tsp vanilla 1/2 tsp baking soda 1 1/2 cup flour 1 1/3 cup chocolate chip can you answer the questions below ? discussion 1. suppose you are given the following amounts of ingredients: 1 dozen eggs 24 tsp. of vanilla 1 lb. (82 tsp.) of salt 1 lb. (84 tsp.) of baking soda 3 cups of chocolate chips 5 lb. (11 cups) of sugar 2 lb. (4 cups) of brown sugar 1 lb. (4 sticks) of margarine a. for each ingredient, calculate how many cookies could be prepared if all of that ingredient were consumed. (for example, the recipe shows that using 1 egg- with the right amounts of the other ingredients- yields 24 cookies. how many cookies can you make if the recipe is increased proportionately for 12 eggs? ) b. to determine the limiting reactant for the new ingredients list, identify which ingredient will result in the fewest number of cookies. c. what is the maximum number of cookies that can be produced from the new amounts of ingredients?
Answers: 1

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3


Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
It is time to practice using potential energy diagrams. Respond to the three questions below on ener...
Questions

Mathematics, 06.03.2020 12:00

Mathematics, 06.03.2020 12:00





Chemistry, 06.03.2020 12:01

Mathematics, 06.03.2020 12:01

Mathematics, 06.03.2020 12:01

English, 06.03.2020 12:01

Mathematics, 06.03.2020 12:02

Mathematics, 06.03.2020 12:02

Computers and Technology, 06.03.2020 12:02

History, 06.03.2020 12:02



Mathematics, 06.03.2020 12:02



Mathematics, 06.03.2020 12:03



