
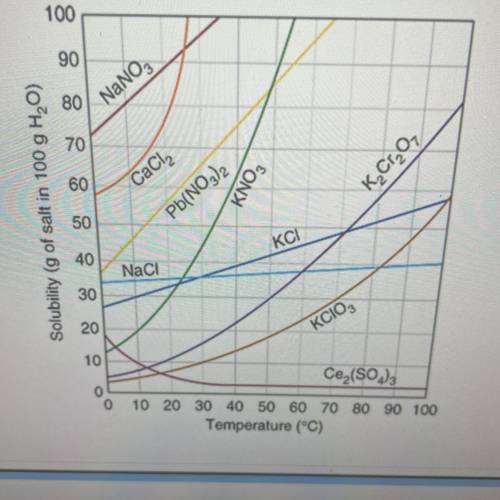
1) what type of solution (saturated or unsaturated ) is present for Pb(NO3)2 if at approximately 25 degrees c
,65 grams of the substance are present in the 100 grams of H2O
2)40 grams of KCl are dissolve in 100 grams of H2O at 10 degrees c how many grams will not dissolve
3)how many grams of H2O are needed to dissolve 50 grams of KClO3 at 70 degrees C
4)how many grams of K2Cr2O7 will dissolve in 75 grams of H2O at 90 degrees C
5) 59 grams of CaCl2 are dissolve in 100 grams of water at approximately 25 degrees c how many more grams of CaCl2 must be added to saturate the solution

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1
You know the right answer?
1) what type of solution (saturated or unsaturated ) is present for Pb(NO3)2 if at approximately 25...
Questions

Mathematics, 05.03.2021 20:20


Physics, 05.03.2021 20:20

Mathematics, 05.03.2021 20:20

Chemistry, 05.03.2021 20:20


Biology, 05.03.2021 20:20

Mathematics, 05.03.2021 20:20





Biology, 05.03.2021 20:20


English, 05.03.2021 20:20


Mathematics, 05.03.2021 20:20


Advanced Placement (AP), 05.03.2021 20:20

Health, 05.03.2021 20:20



