Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

You know the right answer?
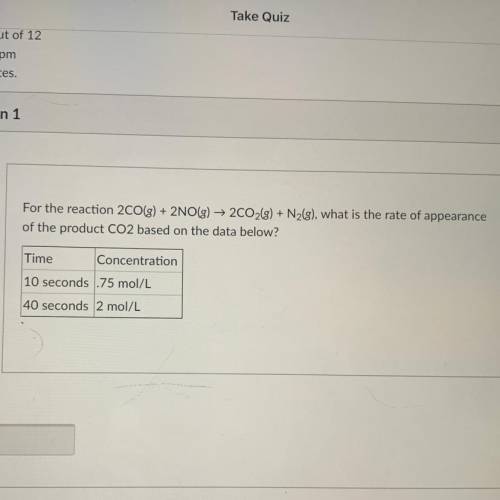
For the reaction 2CO(g) + 2NO(g) → 2C02(g) + Nzg), what is the rate of appearance
of the product CO...
Questions





Social Studies, 01.07.2020 23:01








Mathematics, 01.07.2020 23:01


English, 01.07.2020 23:01



Mathematics, 01.07.2020 23:01