Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1
You know the right answer?
ASAP PLS
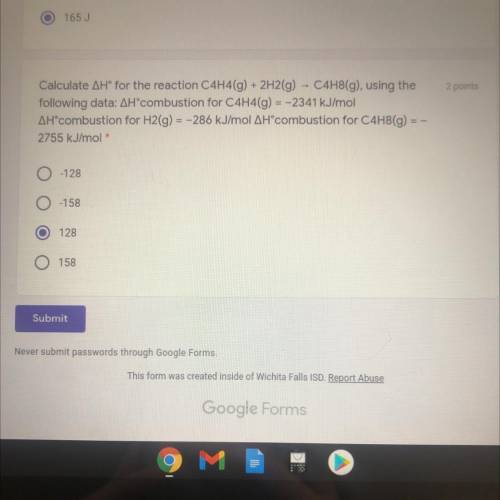
Calculate AH® for the reaction C4H4(g) + 2H2(g) - C4H8(g), using the
following data:...
following data:...
Questions

Mathematics, 28.09.2020 21:01

Social Studies, 28.09.2020 21:01



Mathematics, 28.09.2020 21:01


Health, 28.09.2020 21:01

Biology, 28.09.2020 21:01

Mathematics, 28.09.2020 21:01

Health, 28.09.2020 21:01

Mathematics, 28.09.2020 21:01

Mathematics, 28.09.2020 21:01

English, 28.09.2020 21:01



Mathematics, 28.09.2020 21:01




Mathematics, 28.09.2020 21:01