
Chemistry, 12.04.2021 18:10 jameskarbar9p8c9d2
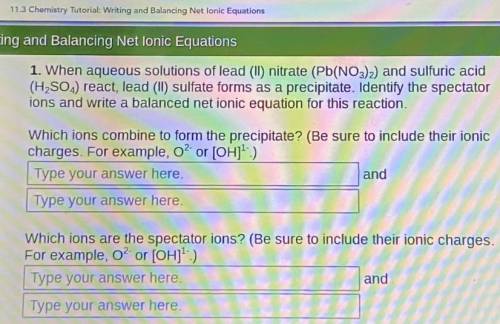
When aqueous solutions of lead (II) nitrate (Pb(NO3)2) and sulfuric acid (H, SO.,) react, lead (II) sulfate forms as a precipitate. Identify the spectator ions and write a balanced net ionic equation for this reaction.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1


Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2
You know the right answer?
When aqueous solutions of lead (II) nitrate (Pb(NO3)2) and sulfuric acid (H, SO.,) react, lead (II)...
Questions

English, 05.05.2020 05:46



English, 05.05.2020 05:46

Spanish, 05.05.2020 05:46


Mathematics, 05.05.2020 05:46

Mathematics, 05.05.2020 05:46






Mathematics, 05.05.2020 05:46


Chemistry, 05.05.2020 05:46

Social Studies, 05.05.2020 05:46

Mathematics, 05.05.2020 05:46

Business, 05.05.2020 05:46




