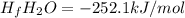A scientist measures the standard enthalpy change for the following reaction to be 67.9 kJ:
Fe2O3(s) + 3 H2(
92Fe(s) + 3 H2O(9)
Based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation
of H2O(g) is
kJ/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 23.06.2019 05:00
Match the term to its description match term definition chemical energy a) internal energy caused by vibrations of atoms and molecules electrical energy b) electromagnetic energy that travels in waves radiant energy c) the movement of an electrical charge thermal energy d) potential energy stored in the bonds between atoms
Answers: 1

You know the right answer?
A scientist measures the standard enthalpy change for the following reaction to be 67.9 kJ:
Fe2O3(s...
Questions

Mathematics, 11.04.2020 02:49




English, 11.04.2020 02:49


Mathematics, 11.04.2020 02:49

Mathematics, 11.04.2020 02:49

Mathematics, 11.04.2020 02:49


Mathematics, 11.04.2020 02:49

History, 11.04.2020 02:49


Chemistry, 11.04.2020 02:49

History, 11.04.2020 02:49





History, 11.04.2020 02:50

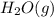 is -252.1 kJ/mol.
is -252.1 kJ/mol.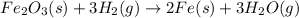
![\Delta H=[n\times H_f_{products}]-[n\times H_f_{reactantss}]](/tpl/images/1252/1104/6418d.png)
![\Delta H=[2\times H_f{Fe}+3\times H_f{H_2O}]-[1\times H_f{Fe_2O_3}+3\times H_f{H_2}]](/tpl/images/1252/1104/83e3e.png)
![67.9kJ=[(2\times 0)+(3\times H_f{H_2O})]-[(1\times -824.2kJ/mol)+3\times 0kJ/mol)]](/tpl/images/1252/1104/4a92c.png)