
Chemistry, 12.04.2021 08:20 shadoris26
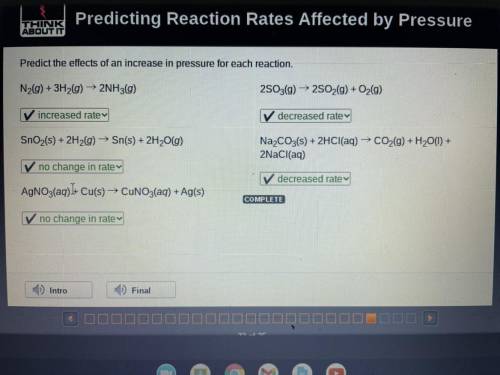
Predict the effects of an increase in pressure for each reaction.
N2(g) + 3H2(g) → 2NH3(g) 2S03(g) ► 2S02(g) + O2(g)
SnO2(s) + 2H2(g) → Sn(s) + 2H20(g)
Na2CO3(s) + 2HCl(aq) → CO2(g) + H20(1) +
2NaCl(aq)
AgNO3(aq))+ Cu(s) → CUNO3(aq) + Ag(s)
COMPLETE

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 23.06.2019 08:30
According to the passage, which of these is true about gray water systems? a) gray water systems use plants that require less water. eliminate b) gray water systems require the use of less fossil fuels. c) gray water systems reduce the amount of fresh water used. d) gray water systems reduce the amount water used by shower heads.
Answers: 1

Chemistry, 23.06.2019 09:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate according to the following equation:
Answers: 2
You know the right answer?
Predict the effects of an increase in pressure for each reaction.
N2(g) + 3H2(g) → 2NH3(g) 2S03(g)...
Questions

Biology, 21.01.2020 20:31

Mathematics, 21.01.2020 20:31


Mathematics, 21.01.2020 20:31


Mathematics, 21.01.2020 20:31

History, 21.01.2020 20:31

History, 21.01.2020 20:31

Advanced Placement (AP), 21.01.2020 20:31




History, 21.01.2020 20:31

Mathematics, 21.01.2020 20:31


Mathematics, 21.01.2020 20:31

English, 21.01.2020 20:31





