
Chemistry, 21.10.2019 17:40 allieb12334
If 143 grams of chromium react with an excess of oxygen, as shown in the balanced chemical equation below, how many grams of chromium oxide can be formed? show all your work for the calculations for full credit.
4cr + 3o2 yields 2cr2o3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1


Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2

Chemistry, 23.06.2019 06:20
Type the correct answer in each box.balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1
You know the right answer?
If 143 grams of chromium react with an excess of oxygen, as shown in the balanced chemical equation...
Questions

Mathematics, 26.06.2019 21:50



History, 26.06.2019 21:50



Health, 26.06.2019 21:50

Mathematics, 26.06.2019 21:50



Computers and Technology, 26.06.2019 21:50

Mathematics, 26.06.2019 21:50


English, 26.06.2019 21:50






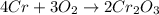
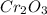 = 152g/mol
= 152g/mol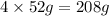 of Chromium produces
of Chromium produces 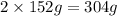 of
of 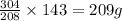 of Chromium oxide(
of Chromium oxide(

