What type of reaction is being shown in this energy diagram?
Energy
Products
Activation...

Chemistry, 11.04.2021 20:20 Kinglilray998
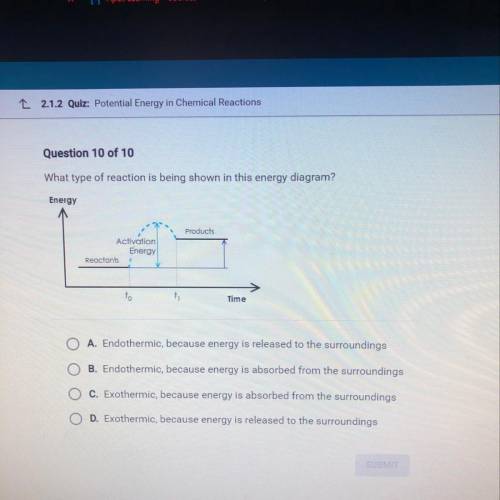
What type of reaction is being shown in this energy diagram?
Energy
Products
Activation
Energy
Reactants
to
ti
Time
A. Endothermic, because energy is released to the surroundings
B. Endothermic, because energy is absorbed from the surroundings
ОО
O
C. Exothermic, because energy is absorbed from the surroundings
D. Exothermic, because energy is released to the surroundings

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1
You know the right answer?
Questions


Mathematics, 18.12.2019 06:31


Mathematics, 18.12.2019 06:31


Mathematics, 18.12.2019 06:31



History, 18.12.2019 06:31


Mathematics, 18.12.2019 06:31


Mathematics, 18.12.2019 06:31


Chemistry, 18.12.2019 06:31


Social Studies, 18.12.2019 06:31

English, 18.12.2019 06:31


Mathematics, 18.12.2019 06:31



