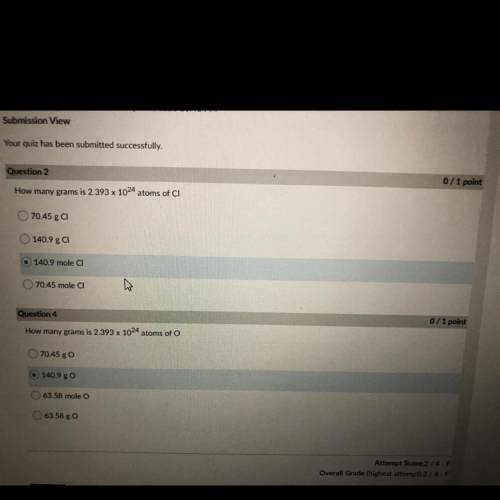
How many grams is 2.393 x 10^24 atoms of CI
70.45 g CI
140.9 ga
140.9 mole CI
70....


Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2
You know the right answer?
Questions




Mathematics, 21.09.2021 16:20

Chemistry, 21.09.2021 16:20

Mathematics, 21.09.2021 16:20



English, 21.09.2021 16:20




Mathematics, 21.09.2021 16:20




Mathematics, 21.09.2021 16:20