
Chemistry, 11.04.2021 08:40 susannaking5852
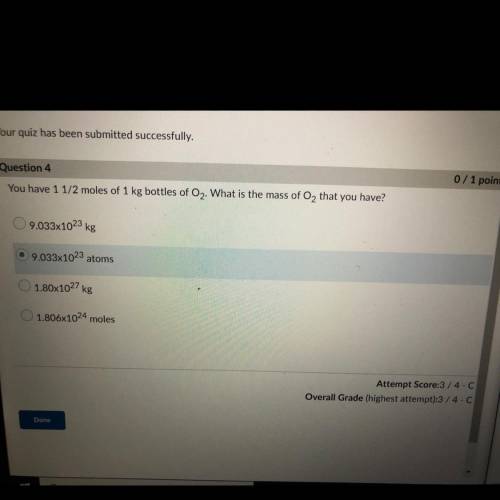
You have 1 1/2 moles of 1 kg bottles of O2. What is the mass of O2 that you have?
A. 9.033x10^23 kg
B. 9.033x10^23 atoms
C. 1.80x10^27 kg
D. 1.806x10^24 moles
It is not B as I have already tried it :(

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
You have 1 1/2 moles of 1 kg bottles of O2. What is the mass of O2 that you have?
A. 9.033x10^23 kg...
Questions



English, 30.10.2020 18:20

Mathematics, 30.10.2020 18:20

Physics, 30.10.2020 18:20

Chemistry, 30.10.2020 18:20

Biology, 30.10.2020 18:20


Mathematics, 30.10.2020 18:20







English, 30.10.2020 18:20

Mathematics, 30.10.2020 18:20

History, 30.10.2020 18:20


Physics, 30.10.2020 18:20



