
Chemistry, 10.04.2021 01:10 mendezmarco2004
A 112g sample of CO(g) is combined with a 32g sample of O2(g) and the reaction represented proceeds as completely as possible. Which TWO of the following statements are correct? *
A. CO(g) is the limiting reactant.
B. O2(g) is the limiting reactant.
C. 4mol of CO(g) reacts.
D. 2mol of CO(g) remains unreacted.
E. 144g of CO2(g) is produced.
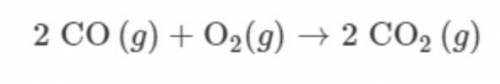

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2


Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2
You know the right answer?
A 112g sample of CO(g) is combined with a 32g sample of O2(g) and the reaction represented proceeds...
Questions



Spanish, 12.03.2020 06:45


History, 12.03.2020 06:45








Mathematics, 12.03.2020 06:46



Mathematics, 12.03.2020 06:46






