H2CO: → H2O + CO2
Answer the following question about the equation above.
1. How many atoms o...

Chemistry, 09.04.2021 06:10 HtetPaing9281
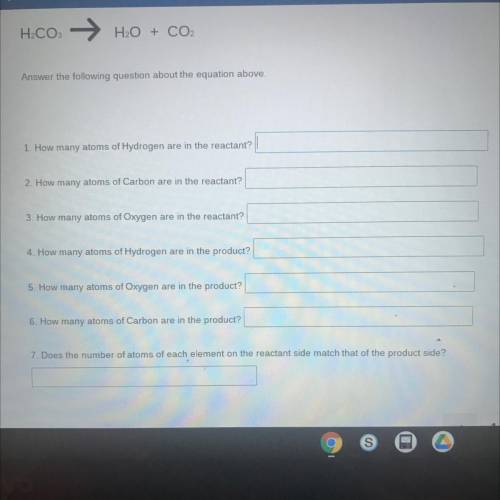
H2CO: → H2O + CO2
Answer the following question about the equation above.
1. How many atoms of Hydrogen are in the reactant?
2. How many atoms of Carbon are in the reactant?
3. How many atoms of Oxygen are in the reactant?
4. How many atoms of Hydrogen are in the product?
5. How many atoms of Oxygen are in the product?
6. How many atoms of Carbon are in the product?
7. Does the number of atoms of each element on the reactant side match that of the product side?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
Questions




History, 18.10.2020 01:01

Geography, 18.10.2020 01:01

Mathematics, 18.10.2020 01:01

English, 18.10.2020 01:01





History, 18.10.2020 01:01



Mathematics, 18.10.2020 01:01


History, 18.10.2020 01:01



Mathematics, 18.10.2020 01:01



