
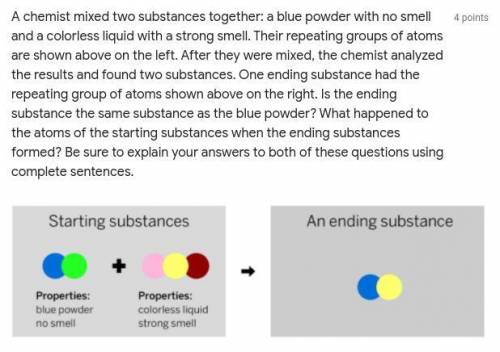
A chemist mixed two substances together: a blue powder with no smell and a colorless liquid with a strong smell. Their repeating groups of atoms are shown above on the left. After they were mixed, the chemist analyzed the results and found two substances. One ending substance had the repeating group of atoms shown above on the right. Is the ending substance the same substance as the blue powder? What happened to the atoms of the starting substances when the ending substances formed? Be sure to explain your answers to both of these questions using complete sentences.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 23.06.2019 01:00
The time that is taken by neptune once around the sun is called
Answers: 1
You know the right answer?
A chemist mixed two substances together: a blue powder with no smell and a colorless liquid with a s...
Questions



Mathematics, 29.01.2020 09:40



Mathematics, 29.01.2020 09:40


Biology, 29.01.2020 09:40

Mathematics, 29.01.2020 09:40





History, 29.01.2020 09:40

Biology, 29.01.2020 09:40


Mathematics, 29.01.2020 09:40

History, 29.01.2020 09:41


History, 29.01.2020 09:41



