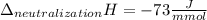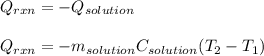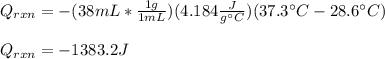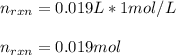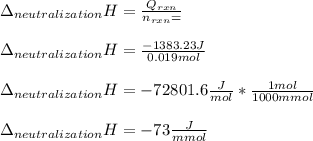Determine the enthalpy of neutralization in Joules/mmol for a solution resulting from 19 mL of 1 M NaOH solution and 19 mL of a HCl with the same molarity. If separately, each had a temperature of 28.6 degrees Celsius, and upon addition, the highest temperature reached by the solution was graphically determined to be 37.3 degrees Celsius. Round to the nearest whole number.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
Determine the enthalpy of neutralization in Joules/mmol for a solution resulting from 19 mL of 1 M N...
Questions




Computers and Technology, 30.08.2019 05:20








Medicine, 30.08.2019 05:20



Computers and Technology, 30.08.2019 05:20




Social Studies, 30.08.2019 05:20