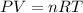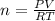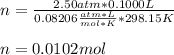Chemistry, 08.04.2021 08:30 reeeeeee32
How many moles would be present in a gas contained in a 100.0 mL vessel at 25.0oC at a pressure of 2.50 atm?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1
You know the right answer?
How many moles would be present in a gas contained in a 100.0 mL vessel at 25.0oC at a pressure of 2...
Questions

History, 10.08.2021 05:00


Mathematics, 10.08.2021 05:00


Mathematics, 10.08.2021 05:00

Mathematics, 10.08.2021 05:00



Chemistry, 10.08.2021 05:00









Business, 10.08.2021 05:00