
Chemistry, 08.04.2021 01:40 Raechelrae04
If an equilibrium mixture of the three gases at 600K contains 2.92*10^-2 M COCH(g) and 1.76*10^2 M CO, what is the equilibrium concentration of Cl2?
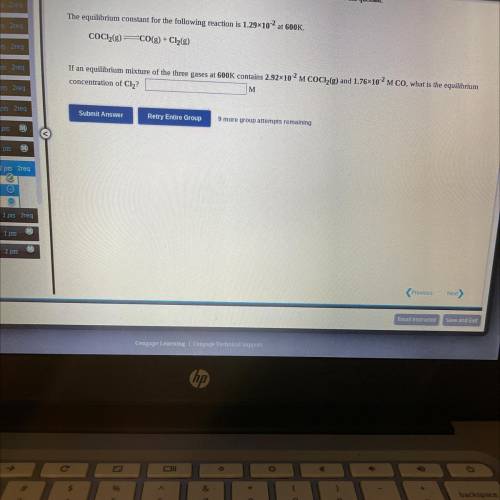

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
The isotonic saline solution described in part a is connected to an unknown solution via a semipermeable membrane, the unknown solution level drops. based on this information, what can be said about these two solutions?
Answers: 1

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
If an equilibrium mixture of the three gases at 600K contains 2.92*10^-2 M COCH(g) and 1.76*10^2 M C...
Questions

Biology, 27.05.2021 01:40


History, 27.05.2021 01:40

Biology, 27.05.2021 01:40





Mathematics, 27.05.2021 01:40



Mathematics, 27.05.2021 01:40


Health, 27.05.2021 01:40

Mathematics, 27.05.2021 01:40


Mathematics, 27.05.2021 01:40

Physics, 27.05.2021 01:40




