
Chemistry, 08.04.2021 01:20 emmaja121003
For each of the seven metal cations (K+, Ba2+, Zn2+, Mn2+, Co2+, Ni2+, Fe3+), write
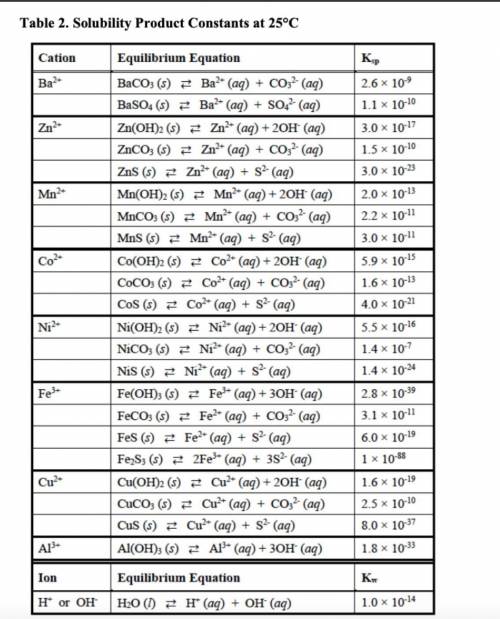
out the corresponding solubility equilibrium equations that would give rise to a precipitate with ammonia. Be careful to use the appropriate arrows (⇄ vs. →), depending on the compound’s solubility. Using the Ksp values provided in Table 2, predict whether it would form using 5 drops (0.25 mL) of 3 M NH3 and 1 mL of 0.10 M metal nitrate solution.
Your answer must include:
• The relevant chemical equation(s) showing dissociation or equilibrium of the
products into their ionic components.
• The Ksp values and equations
• Calculations showing how concentrations were determined
• Unique Qsp calculations
• A comparison of Qsp and Ksp, and discussion of what this comparison predicts
about precipitate (solid) formation.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 23.06.2019 07:00
Why do the strengths of london (dispersion) forces generally increase with increasing molecular size? choose one: a. heavier atoms have stronger attractions for each other than lighter atoms. b. dispersion forces are all equal in magnitude; there is no size dependence. c. dispersion forces arise from the attraction between the nuclei of atoms, and larger molecules have larger nuclei. d. dispersion forces arise from dipoles caused by the electron distribution being distorted. larger molecules have more electrons and, therefore, more distortions and a bigger force. e. dispersion forces depend on distance. larger molecules are farther apart and so the forces are smaller.
Answers: 2
You know the right answer?
For each of the seven metal cations (K+, Ba2+, Zn2+, Mn2+, Co2+, Ni2+, Fe3+), write
out the corresp...
Questions




History, 14.07.2019 16:30


Mathematics, 14.07.2019 16:30

Mathematics, 14.07.2019 16:30




Biology, 14.07.2019 16:30


History, 14.07.2019 16:30



Mathematics, 14.07.2019 16:30

English, 14.07.2019 16:30

Mathematics, 14.07.2019 16:30




