
Chemistry, 22.09.2019 17:30 devbar3416
When a given reaction is conducted in a calorimeter, energy is absorbed from the surrounding water that results in a decrease in the water’s temperature. which of the following potential energy diagrams best illustrates the energy change of this dissolving process?
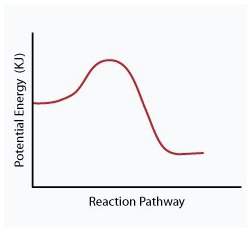

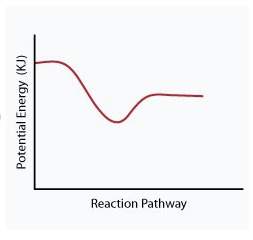
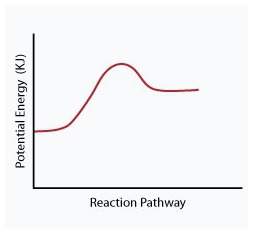
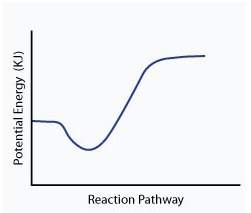

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

You know the right answer?
When a given reaction is conducted in a calorimeter, energy is absorbed from the surrounding water t...
Questions


Chemistry, 18.02.2021 17:20

Biology, 18.02.2021 17:20

Mathematics, 18.02.2021 17:20

Biology, 18.02.2021 17:20

Mathematics, 18.02.2021 17:20


Mathematics, 18.02.2021 17:20

Physics, 18.02.2021 17:20



Mathematics, 18.02.2021 17:20






Mathematics, 18.02.2021 17:20


English, 18.02.2021 17:20



