
Chemistry, 07.04.2021 19:50 nyasiasaunders1234
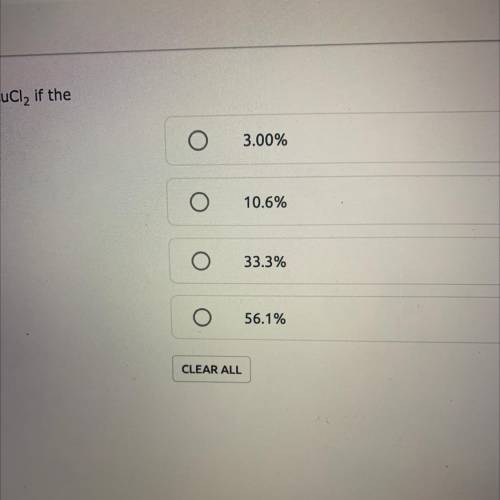
A reaction yields 6.26 grams of a CuCl2. What is the percent yield of CuCl2 if the theoretical yield is 18.81g? % Yield = (Actual Yield/Theoretical Yield) x 100

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3
You know the right answer?
A reaction yields 6.26 grams of a CuCl2. What is the percent yield of CuCl2 if the theoretical yield...
Questions

Mathematics, 23.02.2021 18:50


Chemistry, 23.02.2021 18:50

History, 23.02.2021 18:50



Mathematics, 23.02.2021 18:50

Geography, 23.02.2021 18:50

Mathematics, 23.02.2021 18:50

Mathematics, 23.02.2021 18:50


Health, 23.02.2021 18:50


Advanced Placement (AP), 23.02.2021 18:50

Mathematics, 23.02.2021 18:50

Mathematics, 23.02.2021 18:50

Mathematics, 23.02.2021 18:50

Mathematics, 23.02.2021 18:50





