
Chemistry, 07.04.2021 18:00 jellyangie1
(Please help 15 points the assignment is late) (multiple questions) (everything is virtual task )
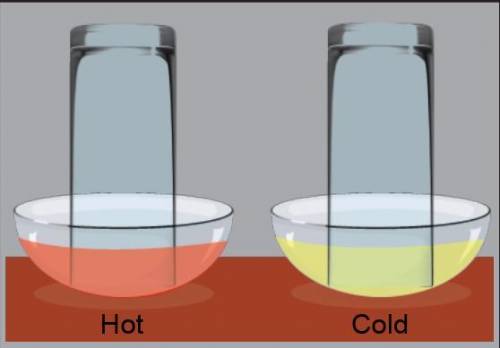
Part 1 What do you think will happen when an effervescent tablet is placed in the water under each inverted glass?
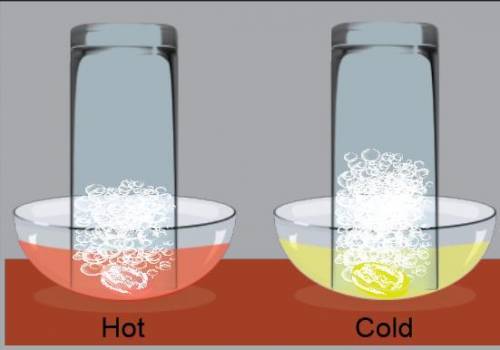
Part 2 Note the changes in the water level and the air space in both glasses, and write down your observations.
Part 3 Did your prediction match your observation?
Part 4. When a substance easily dissolves in a liquid, it has high solubility in that liquid. When a substance does not easily dissolve in a liquid, it has low solubility in that liquid. Solubility of a gas in water describes how well the liquid can “hang on” to gas, instead of releasing it into the air. Based on the results of your experiment, do you think that CO2 has a higher solubility in hot water or cold water? Why?
Part 5 Soda pop is carbonated with CO2. Mark puts one bottle of soda pop in the refrigerator and leaves the other out in the hot sunlight. After one hour, he opens both bottles. Which bottle will likely have more fizzing and bubbles? Why?
Part 6 One result of climate change is that ocean temperatures are increasing. If the temperatures continue to rise, what effect will that have on the oceans’ ability to retain CO2? How might this change affect the atmosphere?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

You know the right answer?
(Please help 15 points the assignment is late) (multiple questions) (everything is virtual task )
P...
Questions





Biology, 21.02.2020 02:41


Mathematics, 21.02.2020 02:41


English, 21.02.2020 02:41


Mathematics, 21.02.2020 02:41


Computers and Technology, 21.02.2020 02:41



Mathematics, 21.02.2020 02:41

Mathematics, 21.02.2020 02:41



Biology, 21.02.2020 02:42



