

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:10
Agas mixture with a total pressure of 745 mmhg contains each of the following gases at the indicated partial pressures: co2, 245 mmhg ; ar, 119 mmhg ; and o2, 163 mmhg . the mixture also contains helium gas. part a what is the partial pressure of the helium gas? phe p h e = nothing mmhg request answer part b what mass of helium gas is present in a 10.2-l sample of this mixture at 283 k ? m m = nothing g request answer
Answers: 1

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2
You know the right answer?
34 g of O2 are reacted with excess Cs, causing a production of 199 g of Cs2O. What is the percent yi...
Questions


Mathematics, 19.02.2021 20:30


Biology, 19.02.2021 20:30

Biology, 19.02.2021 20:30

Mathematics, 19.02.2021 20:30




Mathematics, 19.02.2021 20:30


Biology, 19.02.2021 20:30



Chemistry, 19.02.2021 20:30

Arts, 19.02.2021 20:30

Social Studies, 19.02.2021 20:30


Mathematics, 19.02.2021 20:30

Mathematics, 19.02.2021 20:30

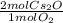 = 2.125 mol Cs₂O
= 2.125 mol Cs₂O

